 Hard water has a much higher concentration of calcium and magnesium that combine to produce limescale. Limescale doesn’t limit itself to well water or mains water. The more calcium and magnesium dissolved in the water, the harder the water becomes. This explains how locations in the same county or area of the country have varying degrees of water hardness.
Now we understand what hard water is, we can start to understand how limescale (hardness) can be removed from our water to make life more pleasurable and in some cases, become life changing.
All salt based water softeners use the process of ion exchange to create soft water from hard.
Hard water has a much higher concentration of calcium and magnesium that combine to produce limescale. Limescale doesn’t limit itself to well water or mains water. The more calcium and magnesium dissolved in the water, the harder the water becomes. This explains how locations in the same county or area of the country have varying degrees of water hardness.
Now we understand what hard water is, we can start to understand how limescale (hardness) can be removed from our water to make life more pleasurable and in some cases, become life changing.
All salt based water softeners use the process of ion exchange to create soft water from hard.
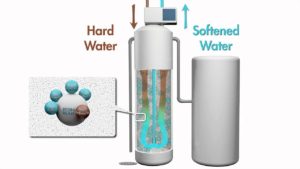 Very simply, the hard water from the water main passes through resin beads contained in a resin tank where the calcium and magnesium are removed and replaced with harmless sodium ions. Scale is literally removed from the water leaving it softened.
The resin beads in the resin tank eventually need cleaning as it becomes unable to pick up any more of the hard minerals. This cleaning process is called ‘regeneration’ and the hard minerals held in the resin are sent to drain. The regeneration process will pass a salt water or brine solution through the resin beads which releases the hardness minerals away to drain. The resin is left clean and ready to go again with the water softening process.
Very simply, the hard water from the water main passes through resin beads contained in a resin tank where the calcium and magnesium are removed and replaced with harmless sodium ions. Scale is literally removed from the water leaving it softened.
The resin beads in the resin tank eventually need cleaning as it becomes unable to pick up any more of the hard minerals. This cleaning process is called ‘regeneration’ and the hard minerals held in the resin are sent to drain. The regeneration process will pass a salt water or brine solution through the resin beads which releases the hardness minerals away to drain. The resin is left clean and ready to go again with the water softening process.